Abstract
Introduction: PI3Kδ inhibition has demonstrated significant efficacy in the treatment of lymphoid malignancies, leading to the approval of idelalisib as a single agent for follicular lymphoma and SLL and in combination with rituximab in CLL. However, therapy is not curative and agents in this class are associated with treatment limiting toxicities (e.g. pneumonitis, colitis, transaminitis). Clinical trials are investigating novel combinations in efforts to increase response depth while limiting treatment durations to avoid toxicities. Given the unique side effects associated with PI3Kδ inhibition, data is needed to guide use in routine clinical practice and to direct future development of this therapeutic class.
Methods: We report on the cumulative experience of therapy with PI3Kδ inhibitors (idelalisib, duvelisib or INCB040093) administered with or without rituximab or obinutuzumab as well as investigational combinations (+JAK1inhibitor, +SYK inhibitor, or + bendamustine rituximab). Demographic and clinical data were collected on all non-Hodgkin lymphoma patients treated with a PI3Kδ inhibitor at a single institution. Data on all treatment related adverse events (AEs) were graded according to the CTCAE V4.03 criteria. Cause specific cox-proportional hazards models were used to model the risk of grade 3 or 4 AEs associated with treatment regimen, treatment history, and age, in the presence of the competing risk of death.
Results: Seventy-nine patients were treated with a PI3Kδ inhibitor (median age 68 years, 87% relapsed/refractory , 78% on clinical trial) for a median of 3.5 months (range 0.2 to 30). Thirty-four patients were treated with a single agent PI3Kδ inibitor +/- an anti-CD20 antibody (standard group) and 45 patients were treated with a novel PI3Kδ combination (novel group). There were no significant differences in demographics or previous treatment between these two groups.
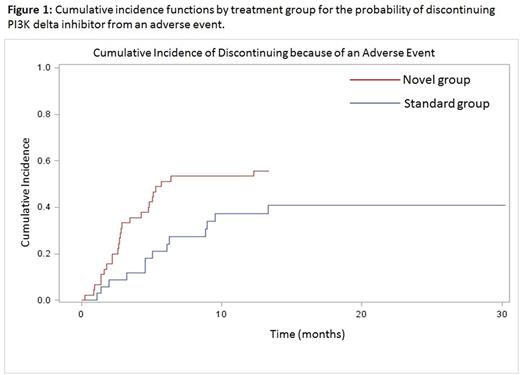
All patients experienced AEs and 68.4% of patients had at least one grade 3/4 (severe) AE, many of which were immune-mediated and infectious events. Two patients died from treatment related toxicity (cause-specific mortality 7%). The incidence rate of severe AEs was significantly higher in the novel combinations group (5.2 vs. 2.1 per person-year; p<0.05). Fifty-four percent of patients discontinued because of an AE. Figure one illustrates a higher cumulative probability of discontinuing from an AE in the novel group, which had a significantly higher risk of discontinuation from AE compared to the standard group (HR 2.8, 95%CI 1.41, 5.76; p<0.01). There was no increase in colitis/diarrhea or hepatic transaminitis in novel combinations compared to standard use. However, we did see a significantly increased risk of pneumonitis in the novel group (HR 4.07, 95% CI 1.23, 13.42; p<0.02) compared to standard use, largely due to use of the idelalisib + SYK inhibitor doublet. The novel group was also at a higher, though non-significant, risk of severe infectious AEs (HR1.81, 95%CI 0.62, 5.93; p>0.05) mostly due to sinopulmonary infections. Previously untreated patients had over two times the risk of a severe AE compared to previously treated patients (HR 2.62, 95%CI 1.19,5.18; p<0.01). There was no evidence of a difference in risk of severe AEs between younger and older patients (HR 1.07, 95%CI 0.62, 1.86, p>0.05).
Conclusions: Several ongoing studies are investigating novel PI3Kδ-based combinations given initial results demonstrated with idelalisib. Our data suggests that the known immune-mediated and infectious toxicities are more common with novel targeted combinations studied to date. Combinations of B-cell signaling inhibitors appear particularly toxic and should be avoided. Phase I investigations should use a traditional conservative dose escalation schema with prolonged monitoring for delayed side effects. Antimicrobial prophylaxis may be needed in future combinatorial investigations. Further, clinical use and future development should focus on relapsed patients given the increased risk of immune-mediated events in untreated patients.
Casulo: Gilead: Honoraria, Other: travel support; Infinity: Consultancy; Celgene: Research Funding. Reagan: Seattle Genetics: Research Funding. Friedberg: Bayer HealthCare Pharmaceuticals.: Other: Data and Safety Monitoring Board: Bayer HealthCare Pharmaceuticals.. Barr: Seattle Genetics: Consultancy; Gilead: Consultancy; Infinity: Consultancy; Celgene: Consultancy; Novartis: Consultancy; AbbVie: Consultancy, Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal